Most health expert recommend six month of exclusive breastfeeding but statics suggest that numbers are not good, almost 95% mothers start breastfeeding but this number drops to 40% in first three month and further it drops to 15% till fifth month. Sometime its due to need of medication usage. Because of these statics its important to provide good information on safety of drugs in breastfeeding so that it can be improved when possible. In this FAQ sheet we will discuss about exposure to Wellbutrin Xl | Bupropion Hydrochloride Tablet, Extended Release while breastfeeding. We will also discuss about common side effects and warnings associated with Wellbutrin Xl | Bupropion Hydrochloride Tablet, Extended Release.
What is Wellbutrin Xl | Bupropion Hydrochloride Tablet, Extended Release used for?
Major Depressive Disorder WELLBUTRIN XL is indicated for the treatment of major depressive disorder. The efficacy of bupropion in the treatment of a major depressive episode was established in two 4-week controlled trials of inpatients and in one 6-week controlled trial of outpatients whose diagnoses corresponded most closely to the Major Depression category of the APA Diagnostic and Statistical Manual (DSM) (see CLINICAL TRIALS). A major depressive episode (DSM-IV) implies the presence of 1) depressed mood or 2) loss of interest or pleasure; in addition, at least 5 of the following symptoms have been present during the same 2-week period and represent a change from previous functioning: depressed mood, markedly diminished interest or pleasure in usual activities, significant change in weight and/or appetite, insomnia or hypersomnia, psychomotor agitation or retardation, increased fatigue, feelings of guilt or worthlessness, slowed thinking or impaired concentration, a suicide attempt, or suicidal ideation. The efficacy of bupropion in maintaining an antidepressant response for up to 44 weeks following 8 weeks of acute treatment was demonstrated in a placebo-controlled trial with the sustained-release formulation of bupropion (see CLINICAL TRIALS). Nevertheless, the physician who elects to use WELLBUTRIN XL for extended periods should periodically reevaluate the long-term usefulness of the drug for the individual patient. Seasonal Affective Disorder WELLBUTRIN XL is indicated for the prevention of seasonal major depressive episodes in patients with a diagnosis of seasonal affective disorder. The efficacy of WELLBUTRIN XL for the prevention of seasonal major depressive episodes was established in 3 controlled trials of adult outpatients with a history of major depressive disorder with an autumn-winter seasonal pattern as defined by Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) criteria (see CLINICAL TRIALS). Seasonal affective disorder is characterized by recurrent major depressive episodes, most commonly occurring during the autumn and/or winter months. Episodes may last up to 6 months in duration, typically beginning in the autumn and remitting in the springtime. Although patients with seasonal affective disorder may have depressive episodes during other times of the year, the diagnosis of seasonal affective disorder requires that the number of seasonal episodes substantially outnumber the number of non-seasonal episodes during the individual's lifetime.
Can I continue breastfeeding if I am using Wellbutrin Xl | Bupropion Hydrochloride Tablet, Extended Release? How long does it stays in breast milk?
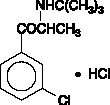
Wellbutrin Xl | Bupropion Hydrochloride Tablet, Extended Release contains Bupropion hydrochloride as active ingredients, . We do not have safety rating of Bupropion hydrochloride but we do have analysis of Bupropion hydrochloride, which is used in manufacturing of Wellbutrin Xl | Bupropion Hydrochloride Tablet, Extended Release. You can get a good idea about Wellbutrin Xl | Bupropion Hydrochloride Tablet, Extended Release usage while breastfeeding by going through our detailed analysis as below.
Statement of Manufacturer/Labeler about breastfeeding usage
Nursing Mothers Like many other drugs, bupropion and its metabolites are secreted in human milk. Because of the potential for serious adverse reactions in nursing infants from WELLBUTRIN XL Tablets, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Wellbutrin Xl | Bupropion Hydrochloride Tablet, Extended Release Breastfeeding Analsys
CAS Number: 34911-55-2
Limited information indicates that maternal bupropion doses of up to 300 mg daily produce low levels in breastmilk and would not be expected to cause any adverse effects in breastfed infants. However, there is little reported use in breastfed newborn infants and case reports of a possible seizure in partially breastfed 6-month-olds. If bupropion is required by a nursing mother, it is not a reason to discontinue breastfeeding. However, another drug may be preferred, especially while nursing a newborn or preterm infant. Infants exposed to bupropion and an SSRI through breastfeeding should be closely monitored for vomiting, diarrhea, jitteriness, or sedation and possibly measurement of serum levels to rule out toxicity if there is a concern.
What should I do if already breastfed my kid after using Wellbutrin Xl | Bupropion Hydrochloride Tablet, Extended Release?
We are not completely sure about safety of Wellbutrin Xl | Bupropion Hydrochloride Tablet, Extended Release in breastfeeding. We would suggest you to contact your doctor or health care provider and explain your situation with Wellbutrin Xl | Bupropion Hydrochloride Tablet, Extended Release. If you observe anything abnormal with your baby please call 911 or contact emergency services in your area.
My doctor has prescribed me Wellbutrin Xl | Bupropion Hydrochloride Tablet, Extended Release, what should I do?
If your doctor considers Wellbutrin Xl | Bupropion Hydrochloride Tablet, Extended Release safe enough to prescribe for you that means its benefits outweigh its known risks.
If I am using Wellbutrin Xl | Bupropion Hydrochloride Tablet, Extended Release, will my baby need extra monitoring?
Not Sure, Please check with your doctor or lactation consultant.
Who can I talk to if I have questions about usage of Wellbutrin Xl | Bupropion Hydrochloride Tablet, Extended Release in breastfeeding?
US
National Womens Health and Breastfeeding Helpline: 800-994-9662 (TDD 888-220-5446) 9 a.m. and 6 p.m. ET, Monday through Friday
UK
National Breastfeeding Helpline: 0300-100-0212 9.30am to 9.30pm, daily
Association of Breastfeeding Mothers: 0300-330-5453
La Leche League: 0345-120-2918
The Breastfeeding Network supporter line in Bengali and Sylheti: 0300-456-2421
National Childbirth Trust (NCT): 0300-330-0700
Australia
National Breastfeeding Helpline: 1800-686-268 24 hours a day, 7 days a week
Canada
Telehealth Ontario for breastfeeding: 1-866-797-0000 24 hours a day, 7 days a week