Breast milk is superior in nutrition, It provides resistance against infections and allergies, It is naturally sterile. Despite all the advantages of breastfeeding some mothers choose to pause the breastfeeding in fear of harmful effects of medicines passing in breast milk. Are you wondering about breastfeeding and using Sotalol Hydrochloride Sotalol Hydrochloride 1 G ? Know what is Sotalol Hydrochloride Sotalol Hydrochloride 1 G and how it can affect your breast milk and whether Sotalol Hydrochloride Sotalol Hydrochloride 1 G is safe for your kid or not.
What is Sotalol Hydrochloride Sotalol Hydrochloride 1 G used for?
Oral sotalol hydrochloride is indicated for the treatment of documented ventricular arrhythmias, such as sustained ventricular tachycardia, that in the judgment of the physician are life-threatening. Because of the proarrhythmic effects of sotalol (see WARNINGS ), including a 1.5 to 2% rate of torsade de pointes or new VT/VF in patients with either NSVT or supraventricular arrhythmias, its use in patients with less severe arrhythmias, even if the patients are symptomatic, is generally not recommended. Treatment of patients with asymptomatic ventricular premature contractions should be avoided. Initiation of sotalol treatment or increasing doses, as with other antiarrhythmic agents used to treat life-threatening arrhythmias, should be carried out in the hospital. The response to treatment should then be evaluated by a suitable method (e.g., PES or Holter monitoring) prior to continuing the patient on chronic therapy. Various approaches have been used to determine the response to antiarrhythmic therapy, including sotalol. In the ESVEM Trial, response by Holter monitoring was tentatively defined as 100% suppression of ventricular tachycardia, 90% suppression of non-sustained VT, 80% suppression of paired VPCs, and 75% suppression of total VPCs in patients who had at least 10 VPCs/hour at baseline; this tentative response was confirmed if VT lasting 5 or more beats was not observed during treadmill exercise testing using a standard Bruce protocol. The PES protocol utilized a maximum of three extrastimuli at three pacing cycle lengths and two right ventricular pacing sites. Response by PES was defined as prevention of induction of the following: 1) monomorphic VT lasting over 15 seconds; 2) non-sustained polymorphic VT containing more than 15 beats of monomorphic VT in patients with a history of monomorphic VT; 3) polymorphic VT or VF greater than 15 beats in patients with VF or a history of aborted sudden death without monomorphic VT; and 4) two episodes of polymorphic VT or VF of greater than 15 beats in a patient presenting with monomorphic VT. Sustained VT or NSVT producing hypotension during the final treadmill test was considered a drug failure In a multicenter open-label long-term study of sotalol in patients with life-threatening ventricular arrhythmias which had proven refractory to other antiarrhythmic medications, response by Holter monitoring was defined as in ESVEM. Response by PES was defined as non-inducibility of sustained VT by at least double extrastimuli delivered at a pacing cycle length of 400 msec. Overall survival and arrhythmia recurrence rates in this study were similar to those seen in ESVEM, although there was no comparative group to allow a definitive assessment of outcome. Antiarrhythmic drugs have not been shown to enhance survival in patients with ventricular arrhythmias. Sotalol is also indicated for the maintenance of normal sinus rhythm [delay in time to recurrence of atrial fibrillation/atrial flutter (AFIB/AFL)] in patients with symptomatic AFIB/AFL who are currently in sinus rhythm and is marketed under the brand name BETAPACE AF™. Sotalol Hydrochloride Tablets are not approved for the AFIB/AFL indication and should not be substituted for BETAPACE AF™ because only BETAPACE AF™ is distributed with a patient package insert that is appropriate for patients with AFIB/AFL.
Is using Sotalol Hydrochloride Sotalol Hydrochloride 1 G unsafe in breastfeeding? Can there be bad consequences for baby if I use it while breastfeeding?
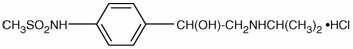
Sotalol Hydrochloride Sotalol Hydrochloride 1 G contains only one active ingredient that is Sotalol hydrochloride. We have analyzed the usage of Sotalol hydrochloride in breastfeeding and our analysis suggest that Sotalol hydrochloride poses Low risk for infant while breastfeeding and hence Sotalol Hydrochloride Sotalol Hydrochloride 1 G itself shall be considered Low risk item for breastfeeding.
Statement of Manufacturer/Labeler about breastfeeding usage
Nursing Mothers Sotalol is excreted in the milk of laboratory animals and has been reported to be present in human milk. Because of the potential for adverse reactions in nursing infants from sotalol, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Sotalol Hydrochloride Sotalol Hydrochloride 1 G Breastfeeding Analsys
Low RiskCAS Number: 3930-20-9
No adverse effects have been reported, but check-up for the possibility of sedation, hypotension, hypoglycemia or bradycardia.
Sotalol Hydrochloride Sotalol Hydrochloride 1 G Breastfeeding Analsys - 2
CAS Number: 3930-20-9
Because of its extensive excretion into breastmilk, its renal excretion and minimal safety data in breastfed infants, other beta-adrenergic blocking drugs are preferred to sotalol, especially while nursing a newborn or preterm infant. Some authors recommend using sotalol during breastfeeding only while monitoring the infant closely for signs of beta-blockade.[1] Infants over 2 months of age have more mature kidney function and are less likely to be affected by sotalol in milk.
What if I already have used Sotalol Hydrochloride Sotalol Hydrochloride 1 G?
During whole lactation period you shall first discuss with your doctor and then together you shall decide whether you shall take that drug or not however if you have already taken Sotalol Hydrochloride Sotalol Hydrochloride 1 G then you shall inform your doctor, But you should not be worried too much as Sotalol Hydrochloride Sotalol Hydrochloride 1 G comes in category of low risk drug.
My health care provider has asked me to use Sotalol Hydrochloride Sotalol Hydrochloride 1 G, what to do?
Though Sotalol Hydrochloride Sotalol Hydrochloride 1 G dose not comes in category of safe drugs rather it comes in category of low risk but if your doctor is aware that you are breastfeeding your baby and has still recommended it then its advantages must be outweighing the risks.
If I am using Sotalol Hydrochloride Sotalol Hydrochloride 1 G, will my baby need extra monitoring?
Not much
Who can I talk to if I have questions about usage of Sotalol Hydrochloride Sotalol Hydrochloride 1 G in breastfeeding?
US
National Womens Health and Breastfeeding Helpline: 800-994-9662 (TDD 888-220-5446) 9 a.m. and 6 p.m. ET, Monday through Friday
UK
National Breastfeeding Helpline: 0300-100-0212 9.30am to 9.30pm, daily
Association of Breastfeeding Mothers: 0300-330-5453
La Leche League: 0345-120-2918
The Breastfeeding Network supporter line in Bengali and Sylheti: 0300-456-2421
National Childbirth Trust (NCT): 0300-330-0700
Australia
National Breastfeeding Helpline: 1800-686-268 24 hours a day, 7 days a week
Canada
Telehealth Ontario for breastfeeding: 1-866-797-0000 24 hours a day, 7 days a week