Nucararxpak | Clindamycin Phosphate,benzoyl Peroxide,cetaphil Kit while Breastfeeding

What is Nucararxpak | Clindamycin Phosphate,benzoyl Peroxide,cetaphil Kit used for?
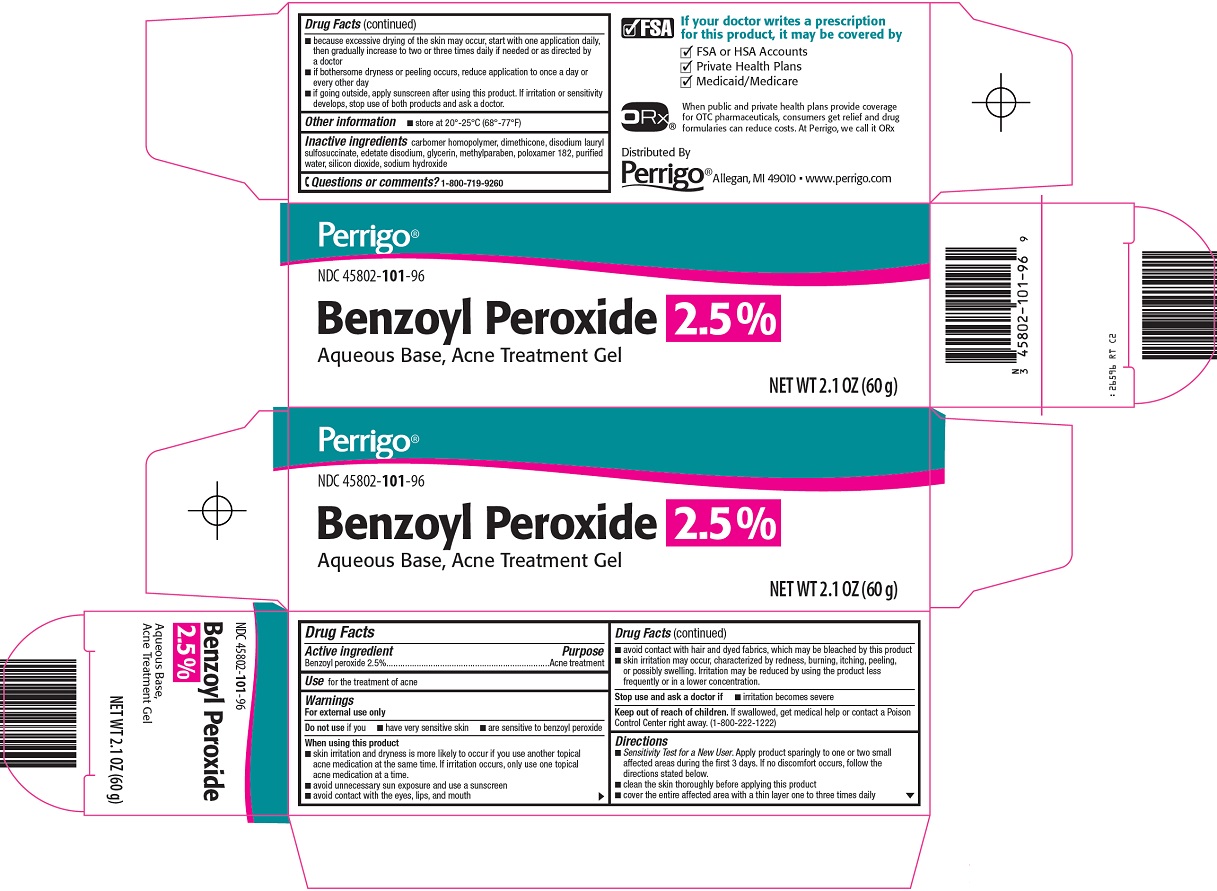
USE for the treatment of acne
USES Helps prevent sunburn.
Brief: Acne treatmentD
Can I continue breastfeeding if I am using Nucararxpak | Clindamycin Phosphate,benzoyl Peroxide,cetaphil Kit? How long does it stays in breast milk?

Nursing Mothers: It is not known whether clindamycin is excreted in human milk following use of clindamycin phosphate. However, orally and parenterally administered clindamycin has been reported to appear in breast milk. Clindamycin has the potential to cause adverse effects on the breastfed infant's gastrointestinal flora. If oral or intravenous clindamycin is required by a nursing mother, it is not a reason to discontinue breastfeeding, but an alternate drug may be preferred. Monitor the infant for possible adverse effects on the gastrointestinal flora, such as diarrhea, candidiasis (thrush, diaper rash) or rarely, blood in the stool indicating possible antibiotic-associated colitis. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for clindamycin and any potential adverse effects on the breastfed child from clindamycin or from the underlying maternal condition.
Nucararxpak | Clindamycin Phosphate,benzoyl Peroxide,cetaphil Kit Breastfeeding Analsys
Benzoyl peroxide while Breastfeeding
SafeCAS Number: 94-36-0
A keratolytic and topical anti-infective agent used in the treatment of acne. Since the last update we have not found published data on its excretion in breast milk Only 5% is absorbed through the skin and is quickly eliminated via urine after being metabolized in the skin to benzoic acid (AEMPS 2013, Seubert 1984, Holzmann 1979), so it is not expected to pass into breast milk.Taken orally, it is destroyed at the gastrointestinal level, so its absorption into milk is zero. It is considered very low risk during breastfeeding (Kong 2013, Worret 2006, Leachman 2006, Zip 2002). Do not apply to the chest in order to prevent the infant from ingesting it; if necessary, apply after breastfeeding and clean well with water before the next feed. Applying topical creams, gels and other products which contain paraffin (mineral oil) to the nipple is to be avoided so that the infant does not absorb them. (Concin 2008, Noti 2003). List of essential medicines WHO: compatible with breastfeeding (WHO / UNICEF 2002).
Clindamycin while Breastfeeding
Low RiskCAS Number: 18323-44-9
Lincosamide antibacterial. Excreted in non-significant level into breast milk. Except for few cases of enterocolitis due to disturbance of intestinal flora no other harm effects have been shown in breastfed infants. All cases spontaneously cured after discontinuation of medication. One reported case (1980) of pseudomembrane colitis in an infant whose mother was on clindamicin and gentamicin. Be aware of the possibility of false negative results of febrile infant bacterial cultures when the mother is on antibiotics and diarrheal disease due to intestinal flora imbalance. The American Academy of Pediatrics rates it as compatible with breastfeeding.
Nucararxpak | Clindamycin Phosphate,benzoyl Peroxide,cetaphil Kit Breastfeeding Analsys - 2
Benzoyl peroxide while Breastfeeding
CAS Number: 94-36-0
Topical benzoyl peroxide has not been studied during breastfeeding. Because only about 5% is absorbed following topical application, it is considered a low risk to the nursing infant.[1][2] Ensure that the infant's skin does not come into direct contact with the areas of skin that have been treated. Only water-miscible cream or gel products should be applied to the breast because ointments may expose the infant to high levels of mineral paraffins via licking.[3]
Clindamycin while Breastfeeding
CAS Number: 18323-44-9
Clindamycin has the potential to cause adverse effects on the breastfed infant's gastrointestinal flora. If oral or intravenous clindamycin is required by a nursing mother, it is not a reason to discontinue breastfeeding, but an alternate drug may be preferred. Monitor the infant for possible effects on the gastrointestinal flora, such as diarrhea, candidiasis (thrush, diaper rash) or rarely, blood in the stool indicating possible antibiotic-associated colitis. Vaginal application is unlikely to cause infant side effects, although about 30% of a vaginal dose is absorbed. Infant side effects are unlikely with topical administration for acne; however, topical application to the breast may increase the risk of diarrhea if it is ingested by the infant. Only water-miscible cream, foam, gel or liquid products should be applied to the breast because ointments may expose the infant to high levels of mineral paraffins via licking.[1]
Nucararxpak | Clindamycin Phosphate,benzoyl Peroxide,cetaphil Kit Breastfeeding Analsys - 3
Octisalate and Breastfeeding
Low Risk
Octyl salicylate is an oil soluble chemical sunscreen agent that absorbs UVB radiation. It does not protect against UVA. Octyl salicylate is used to augment the UVB protection in a sunscreen. Salicylates are weak UVB absorbers and they are generally used in combination with other UV filters
Octisalate rarely causes allergies in tropical usage. Not much study has been done on effects of topical usage of Octisalate during breast feeding however it is known to penetrate the skin hence it�s better to use other alternatives.
FDA study found blood levels 10 times above cutoff for systemic exposure, skin penetration in lab studies has been observed
Note: Study and data for tropical use onlyWarning: Tropical usage in breast area shall be avoided to prevent the Octisalate passing orally in Infants.
Octocrylene and Breastfeeding
Safe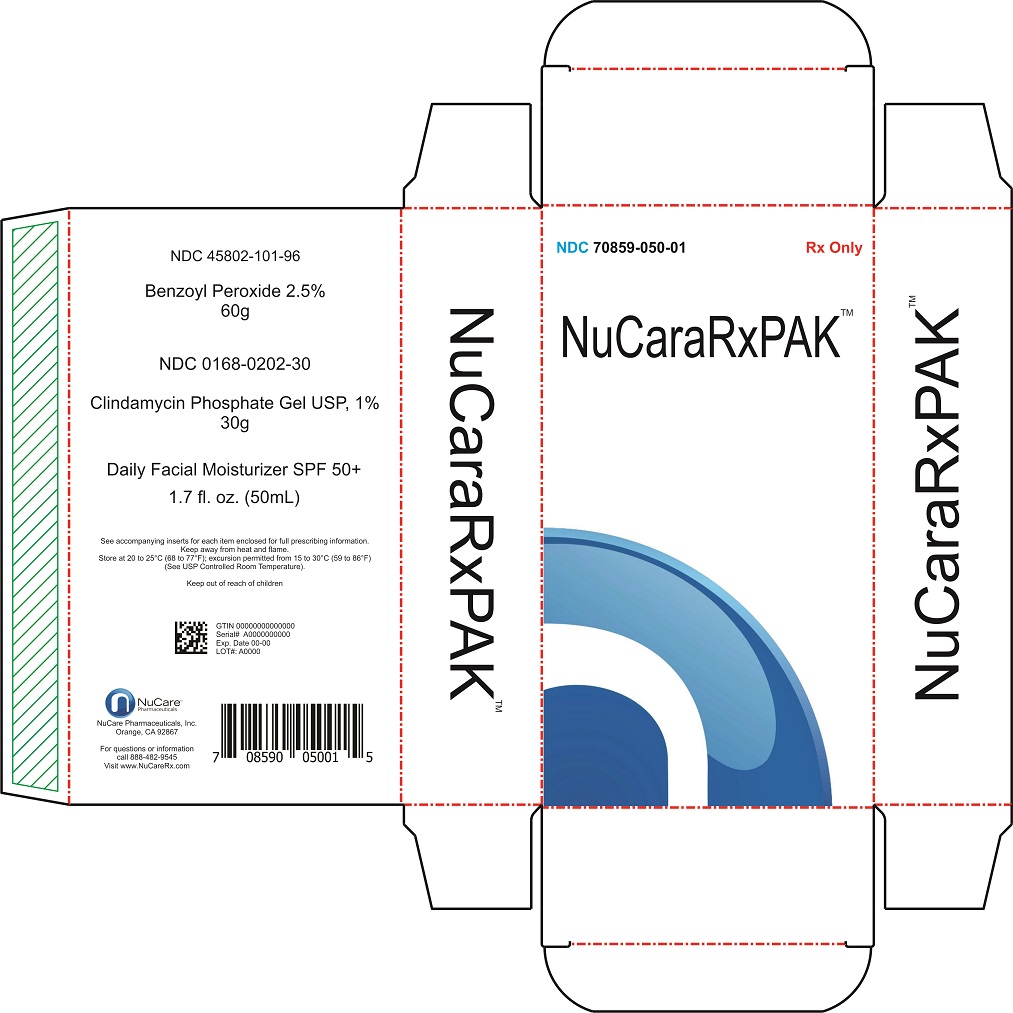
Based on the current available safety data, octocrylene used as a UV filter in cosmetic products at a concentration of 10% can be considered as safe. There was no evidence of any endocrine disruption potential from experimental studies which demonstrated no adverse effects on reproductive (e.g. oestrus cycle, epididymal and testicular sperm parameters) and developmental parameters. Effects on thyroid reported in repeated toxicity studies conducted in rats at very high doses are species?specific and not relevant considering the doses at which octocrylene is used in human.
Four studies on the transdermal absorption of octocrylene are available in the scientific literature, and an additional study is available in ECHA summaries of safety data. Dermal absorption studies of octocrylene showed that most octocrylene concentrations are found in the stratum corneum and that very few quantities are found in the epidermis (0.4%) and in the receptor fluid (<0.05%). In vivo, a very recent study in human volunteers showed systemic exposure to octocrylene with maximal concentrations ranging from 2.9 to 7.8 ng/mL under indoor maximal use conditions. Octocrylene has been found at very low amounts in human milk, and some metabolites of octocrylene were primarily detected in urine of volunteers using sunscreen products.
No systemic effects were reported after dermal exposure to octocrylene in rabbits at very high dose (534 mg/kg bw/day) compared with those used in cosmetic products. After oral exposure, effects on liver and thyroid were reported in a study conducted in rats at high doses (340 and 1085 mg/kg bw/day in males). These effects were investigated in an additional mechanistic study which showed that effects on thyroid were indirect and due to hepatic enzyme induction potential of octocrylene in rats at very high oral doses.
Based on available animal data, octocrylene does not induce developmental or teratogenic effects. In an extended one?generation reproductive toxicity study, only rats treated with the highest dose of octocrylene via oral route showed a decrease in the number of implantation sites and consequently a low number of pups. This very high dose of 550 mg/kg bw/day cannot be considered to be relevant to the dermal use of octocrylene as a cosmetic ingredient. Moreover, no other effects on male and female fertility and reproductive parameters such as oestrus cycle, epididymal and testicular sperm parameters were observed in all groups tested. Regarding pups, no effects on sexual and neurodevelopmental parameters were observed.
Note: Study and data for tropical use onlyWarning: Tropical usage in breast area shall be avoided to prevent the Octocrylene passing orally in Infants. In some rare cases it can cause skin allergy.
Titanium dioxide and Breastfeeding
Low RiskNot much study has been done on effects of topical usage of Titanium Dioxide during breast feeding but as there is no finding of Titanium Dioxide passing in breast milk its unlikely to cause any health issue for infant.
Some animal studies suggest that maternal exposure to titanium dioxide nanoparticles during pregnancy and lactation alters offspring hippocampal mRNA BAX and Bcl-2 levels, induces apoptosis and decreases neurogenesis. But dosage was significantly higher than daily possible exposure to humans.
Note: Study and data for tropical use only. Inhalation concerns in powder or spray products.Warning: Tropical usage in breast area shall be avoided to prevent the Titanium Dioxide passing orally in Infants. Titanium dioxide is possibly carcinogenic to humans. Titanium dioxide can be drastically more harmful if used as powder or spray as risk of inhalation can increase significantly.
Oxybenzone and Breastfeeding
DangerousOxybenzone has been found in mother�s milk. And has relatively high 1% to 9% skin penetration in lab studies. Oxybenzone has relatively high rates of skin allergy, it has weak estrogen like effects, and its observed as moderate anti-androgen. Oxybenzone is associated with altered birth weight in human studies. It is not recommended to use Oxybenzone during breast feeding.
Note: Study and data for tropical use only.Warning: Tropical usage in breast area shall be avoided to prevent the Oxybenzone passing orally in Infants.
Octinoxate and Breastfeeding
UnsafeOctinoxate (Octylmethoxycinnamate) has been detected in human urine, blood and breast milk and is known for moderate risk of skin allergy. Some studies suggest that Octinoxate has estrogen like effects however less than 1% skin penetration has been found in human laboratory studies. As not much study has been done on effects of Octinoxate during breast feeding its recommended to use safe alternatives.
Octyl Methoxycinnamate (OMC) is a frequently used UV-filter in sunscreens and other cosmetics. Octinoxate can be systemically absorbed after skin application, being found in the deeper layers of the stratum corneum as well as urine, plasma, and breast milk. The mean maximum plasma concentration detected after application of 2mg/cm2 sunscreen was 7ng/mL in women and 16ng/mL in men. FDA study found blood levels 13 times above cutoff for systemic exposure.
Several studies indicated that OMC acts as an endocrine disruptor due to the ability to interfere with endocrine system at different levels. In humans OMC exposure has minor, but statistically significant effects on the levels of testosterone and estradiol. Moreover, some studies suggested that OMC can interact with the hypothalamo-pituitary-thyroid (HPT) axis.
Moreover, a study of offspring of dams treated with OMC (500�1000 mg/kg/day) showed sex-dependent behavioral changes, namely decreased motor activity in females, but not in males, and improved spatial learning in males, suggesting that OMC can affect neuronal development, however the doses used in these experiments were extremely high, not relevant to possible human exposure.
Note: Study and data for tropical use onlyWarning: High dosage shall be avoided as reproductive system, thyroid and behavioral alterations in animal studies has been found, Tropical usage in breast area shall be avoided to prevent the OCTINOXATE passing orally in Infants.
What should I do if already breastfed my kid after using Nucararxpak | Clindamycin Phosphate,benzoyl Peroxide,cetaphil Kit?
You should immediately inform your health care provider about Nucararxpak | Clindamycin Phosphate,benzoyl Peroxide,cetaphil Kit usage and your breastfeeding interval after usage of
My doctor has prescribed me Nucararxpak | Clindamycin Phosphate,benzoyl Peroxide,cetaphil Kit, what should I do?
Please double check with your doctor if he is aware of your breastfeeding stratus, Ask your doctor if there is any safe alternative of Nucararxpak | Clindamycin Phosphate,benzoyl Peroxide,cetaphil Kit. Check with your doctor if you shall temporally stop breastfeeding. You may go for second opinion as well. Still after all of this if your doctor still recommends Nucararxpak | Clindamycin Phosphate,benzoyl Peroxide,cetaphil Kit then go for it as they have access on more detailed medical and scientific information and they understand your individual medical situation much better.
If I am using Nucararxpak | Clindamycin Phosphate,benzoyl Peroxide,cetaphil Kit, will my baby need extra monitoring?
Extreme level of monitoring required as Nucararxpak | Clindamycin Phosphate,benzoyl Peroxide,cetaphil Kit could be dangerous for kid.
Who can I talk to if I have questions about usage of Nucararxpak | Clindamycin Phosphate,benzoyl Peroxide,cetaphil Kit in breastfeeding?
US
National Womens Health and Breastfeeding Helpline: 800-994-9662 (TDD 888-220-5446) 9 a.m. and 6 p.m. ET, Monday through Friday
UK
National Breastfeeding Helpline: 0300-100-0212 9.30am to 9.30pm, daily
Association of Breastfeeding Mothers: 0300-330-5453
La Leche League: 0345-120-2918
The Breastfeeding Network supporter line in Bengali and Sylheti: 0300-456-2421
National Childbirth Trust (NCT): 0300-330-0700
Australia
National Breastfeeding Helpline: 1800-686-268 24 hours a day, 7 days a week
Canada
Telehealth Ontario for breastfeeding: 1-866-797-0000 24 hours a day, 7 days a week
