Pollen Weeds while Breastfeeding
What is Pollen Weeds used for?
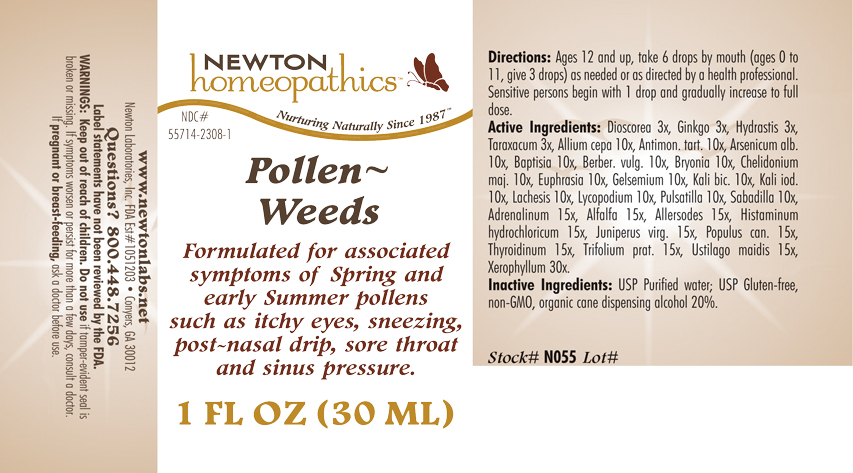
Purpose: OTC - PURPOSE SECTION Formulated for associated symptoms of Spring and early Summer pollens such as itchy eyes, sneezing, post-nasal drip, sore throat and sinus pressure
Is Pollen Weeds usage safe while breastfeeding? If a lactating mother is using it can there be any effect on growth or development of infant?

Pollen Weeds Breastfeeding Analsys
Epinephrine while Breastfeeding
SafeCAS Number: 51-43-4
Used as a systemic drug for cardiac resuscitation and locally in topical anesthetic preparations, epidural anesthesia, eye drops (mydriatic), and nasal drops (vasoconstrictor). At last update no published data on excretion in breast milk were found.Its rapid metabolism (short Tmax and T½) makes it unlikely an excretion into milk in significant amounts. It is destroyed in the gastrointestinal tract. The low oral-bioavailability makes its levels into infant's plasma, that would be absorbed from ingested breast milk, be nil or negligible. Maintained high intravenous doses decrease milk production by interfering with the secretion of prolactin especially the early postpartum-weeks, because when breastfeeding is well established, prolactin levels do not correlate with milk production any longer. No problems related to lactation have been observed due to epidural anesthesia in which adrenaline is used.
Alfalfa while Breastfeeding
UnsafeCAS Number: 8015-60-9
Aerial summits and seeds are used. It contains a great deal of flavonoids, steroids, cumestans, vitamins and minerals Attributed effects but not clinically tested are: agonist of estrogen, antianemic and diuretic. Also, there is not reliable data that would support its use as galactagogue. At latest update, relevant published data on excretion into breast milk were not found. Because its estrogenic effect it should not be consumed during pregnancy and breastfeeding. Caravanina which is one of the components, is toxic if continuously used. Pancytopenia, Hemolytic anemia and Lupus Erythematosus have been described as induced by frequent consumption of germinated-seeds or tablets of alfalfa. Cautious measures before consumption of herbal infusions should include: 1. Make sure that the source is reliable: occurrance of intoxication cases after mistakenly use of a toxic plant, poisoning by consumption of heavy metal containing substances or contaminated food by bacterial or fungal toxins. 2. Avoid excessive use. The “natural products” are not harmless at whatever dosage: the plants contain active substances that have been the source of our common pharmaceutical drugs. They may be a cause of poisoning if consumed in high quantity or for a long time.
Sambucus nigra flowering top while Breastfeeding
Low RiskCAS Number: 977002-47-3
Bush. Bark, leaves, roots, flowers and fruits are used for medicinal purposes in traditional medicine (diuretic, laxative, anti-infective for cold relief ...) without any scientific evidence on effectiveness.It contains flavonoids (quercetin, isoquercitin and rutin), phenolic acids, terpenes, minerals, tannins. Quercetin is excreted in breast milk in a concentration which increases with diets that contain products like elderberry. In the elderberry as well as other plants are contained endocrine disruptors that could display pro or counter-estrogen activity. Since it has no proven therapeutic benefit, it seems wise to avoid any consumption or doing it in very moderate way during lactation.
Populus nigra pollen while Breastfeeding
SafeBuds, leaves and bark are used. Buds contain essential oil, flavonoids and phenolic glycosides. Leaves and bark contain salicin and tannins. Indications after Commission E of German Ministry of Health are: topically as anti-bacterial and healing treatment of burns, wounds and hemorrhoids. Avoid applying it on the breast. Commission E does not recommend it for systemic use. No effectiveness of the otherwise popular use of leaves and bark as diuretic, anti-rheumatic or as a treatment of prostatic hypertrophy has been shown.
Salix alba pollen while Breastfeeding
SafeBark is used. It contains salicylic derivatives (2 – 11%) organic acids (salicylic) flavonoids and tannins. ESCOP and Commission E of German Ministry of Health do recommend it as anti-inflammatory and pain relief agent.
Rosa canina flower while Breastfeeding
SafeCAS Number: 84696-47-9
False fruits (red hips), petals ,and seeds are used It contains ascorbic acid, carotenes, oil, essential oil.. Unproved effects: laxative, diuretic. Indication after Commission E of German Ministry of Health: none. Maximal daily dose: 2 g
Primula vulgaris while Breastfeeding
SafeRoots and flowers are used. Indication after Commission E of German Ministry of Health: Catarrhs of the respiratory tract.This herb is not evening primrose, Oenothera biennis.
Onion while Breastfeeding
SafeCAS Number: 8054-39-5
At latest update no published data on excretion into breast milk were found. It is a widely used plant in preparing food and also in Phytotherapy as herbal extracts or essential oil. Given its lack of toxicity, a moderate consumption as herbal-based medicine during lactation would have low or no risk. Its consumption as a food is devoid of risk. The plant's bulbs are used which contain fructans, polysaccharides, flavonoids (quercetin glycosides), saponins, sterols and sulfoxides. Properties that were approved by the Commission E of the German Ministry of Health are: antibacterial, fat-lowering, antihypertensive, platelet aggregation inhibitor. It is used to treat loss of appetite and topically for bedsores and contractures. It has been used as a topical treatment for pain or inflammation of the nipple.There is insufficient evidence that would suggest ingestion of onions by the mother may be a cause of colicky pain or breast milk rejection by the infants.
Arsenic trioxide while Breastfeeding
DangerousUsed in the treatment of promyelocitic leukemia in adults.
Berberis vulgaris root bark while Breastfeeding
DangerousCAS Number: 84649-92-3
Roots and bark are used. It contains Berberine that may be a cause of gastritis, nephritis, phototoxicity and severe jaundice by displacement of albumin -linked bilirubin: higher risk of kernicterus to newborns, which is greater in cases of 6-Glucose-PD deficiency. It is popularly widely used, however, its effectiveness has not been shown. Use not approved by the Commission E of German Ministry of Health. It should be avoided.
Bryonia alba root while Breastfeeding
Low RiskClimbing plant. The female inflorescences or flower tips are used.It contains phloroglucinols, estrogenic, quercetin, kaempferol, tannins, phenolic acids essential oil and flavonoids. One of its components, 8-prenylnaringenin (8-PN) is the most powerful phytoestrogen known. Properties that are attributed: hypnotic, sedative, orexigenic.It is used as a flavoring and stabilizer of the beer.Indications German Commission E Ministry of Health, EMA and ESCOP: insomnia, nervousness, anxiety There is no scientific evidence showing an improvement in milk production.A possible estrogenic effect may be a decrease in milk production.The best galactogogue is a frequent and on-demand breastfeeding along with proper technique. During breastfeeding its consumption should be moderate or occasional.
Euphrasia stricta while Breastfeeding
SafeCAS Number: 790302-50-0
Aerial summits are used. It contains galic tannins, phenol-carboxylic acids, flavonoids and iridoid heterosides. The Commission E of German Ministry of Health does not support the traditional use as anti-diarrhea and eye anti-inflammatory agent.
Potassium dichromate while Breastfeeding
UnsafeCAS Number: 7778-50-9
Así como el Cromo (véase ficha) en estado trivalente (3+) y a dosis apropiadas carece de toxicidad y constituye un oligoelemento nutricional esencial, las sales hexavalentes (6+) de cromo tienen usos industriales (cromado), son oxidantes, corrosivas, irritantes, están catalogadas como carcinogenos 1A y mutágenos 1B (INSHT 2012, ATSDR 2012), pueden causar dermatitis de contacto y toxicidad crónica y aguda grave.El cromo 6+ se encuentra también en el humo de combustión del tabaco, en cosméticos (Hepp 2014) y en algunas prótesis osteoarticulares (Oppermann 2015). Los compuestos de cromo exavalente, por su peligrosidad, no tienen actualmente usos médicos. A nivel de riesgo laboral para la madre lactante, las frases (INSHT 2008) de riesgo (antiguas frases R, actualmente frases H de Hazard, peligro) o de prudencia (frases P) que deben aparecer en la ficha de seguridad de un producto son sólo dos:- H362 (ant. R64): "Puede perjudicar a los niños alimentados con leche materna"- P263: "Evitar el contacto durante el embarazo y la lactancia"Otras tres frases que se deben considerar durante la lactancia tienen relación con el poder cancerígeno, mutagénico o acumulativo de un producto:- H351 (ant. R40): "Posibles efectos cancerígenos" - H371 (ant. R68): "Posibilidad de efectos irreversibles" - H373 (ant. R33): "Peligro de efectos acumulativos" Basándose en la ausencia de estas frases en las fichas de seguridad del trióxido de cromo (Panreac 2012, INSHT 2005), no sería preciso apartar a la madre lactante de su puesto de trabajo, bastándole a la empresa con cumplir las exigencias legales de valores máximos de exposición ambiental y a la trabajadora las normas aconsejadas de prudencia (guantes, lavado de manos, cambio de ropa, etc. (Panreac 2012). Pero según la normativa europea vigente (Reglamento CE 2008) cuando un producto es carcinógeno y mutágeno, las madres lactantes trabajadoras no deben estar expuestas a mezclas, sean de sólidos, líquidos o gases, con límites de concentración superiores a 0,3%.Además resulta un anomalía el que estando clasificado como cancerígeno 1A, no se le aplique la frase H351 (R40). A la luz de toda la bibliografía, consideramos que la madre lactante no debería estar expuesta a compuestos hexavalentes de cromo en el ambiente laboral.
Potassium iodide while Breastfeeding
SafeCAS Number: 7447-40-7
Human milk has a potassium concentration of 13 meq/L, almost a half of rehydration solution content and a quarter of maximal IV recommended dose. Potassium supplementation does not alter milk concentration without increasing mother’s serum concentration, which is strictly limited from 3,5 to 5,5 meq/L.
Lycopodium clavatum spore while Breastfeeding
UnsafeAerial summits and spores of this fern are used. Traditionally use as a diuretic and intestinal spasm relief drug. Also used for abrasions and skin irritation. It may be a cause of asthma and contact dermatitis.
Ginkgo while Breastfeeding
Low RiskCAS Number: 90045-36-6
Leaves of tree are used.It contains flavonoids, tannins, diterpenes, steroids..Unproved effects: venous tonic, capillary protector, vasodilator (neuron-protector) and platelet anti-aggregationIndications after Commission E of German Ministry of Health: brain vascular insufficiency, intermittent claudication, dizziness, tinnitus. Fluids or solutions with alcoholic content are to be avoided.
Taraxacum officinale while Breastfeeding
SafeThe roots and leaves of the plant in salads both as food and herbal medicine are used.It contains inulin, terpenes, phytosterols, flavonoids, coumarins and potassium salts in large quantities.Attributed Properties: cholagogue, laxative, orexigenic. diureticIndication by the German Commission E Ministry of Health: dyspepsia, cholelithiasis, anorexia.Indications by the European Medicament Agency (EMA): diuretic. Non toxic. There is no evidence of its effectiveness as galactogogue.Best galactogogue is a frequent on-demand breastfeeding and proper technique. Its wide use, low toxicity and since it is also consumed as food, a moderate consumption during lactation is considered of little or no risk. Precaution is recommended before taking herbal infusions:1. Ensure a reliable source: poisoning occurred by confusion with another plant that resulted to be toxic, poisoning from heavy metals and food poisoning by contamination with bacteria or fungi.2. Avoid excessive consumption. The "natural" products are not good in any amount: plants contain active substances from which come out much of our traditional pharmacopoeia and can cause poisoning if eaten in exaggerated quantity or prolonged time.
Pollen Weeds Breastfeeding Analsys - 2
Epinephrine while Breastfeeding
CAS Number: 51-43-4
No information is available on the use of epinephrine during breastfeeding. Because of its poor oral bioavailability and short half-life, any epinephrine in milk is unlikely to affect the infant. High intravenous doses of epinephrine might reduce milk production or milk letdown. Low-dose intramuscular (such as Epi-Pen), epidural, topical, inhaled or ophthalmic epinephrine are unlikely to interfere with breastfeeding. To substantially diminish the effect of the drug after using eye drops, place pressure over the tear duct by the corner of the eye for 1 minute or more, then remove the excess solution with an absorbent tissue.
Alfalfa while Breastfeeding
CAS Number: 8015-60-9
Alfalfa (Medicago sativa) leaves and sprouts contain saponins, estrogenic isoflavinoids (e.g., dianzein genistein), vitamin K, and the amino acid L-canavanine. Alfalfa is a purported galactogogue and is included in some proprietary mixtures promoted to increase milk supply;[1][2][3][4][5] however, no scientifically valid clinical trials support this use. Galactogogues should never replace evaluation and counseling on modifiable factors that affect milk production.[6] Dianzein and genistein are excreted into breastmilk in small amounts,[7][8][9] but have not been measured specifically after alfalfa intake. No data exist on the excretion of other components of alfalfa into breastmilk or on the safety and efficacy of alfalfa in nursing mothers or infants. Alfalfa is generally well tolerated and is "generally recognized as safe" (GRAS) as a food by the U.S. Food and Drug Administration. Worsening of systemic lupus erythematosus has been reported, possibly caused by immune system stimulation by L-canavanine. Because of its vitamin K content, alfalfa should be avoided in persons taking warfarin. Dietary supplements do not require extensive pre-marketing approval from the U.S. Food and Drug Administration. Manufacturers are responsible to ensure the safety, but do not need to the safety and effectiveness of dietary supplements before they are marketed. Dietary supplements may contain multiple ingredients, and differences are often found between labeled and actual ingredients or their amounts. A manufacturer may contract with an independent organization to verify the quality of a product or its ingredients, but that does certify the safety or effectiveness of a product. Because of the above issues, clinical testing results on one product may not be applicable to other products. More detailed information #about dietary supplements# is available elsewhere on the LactMed Web site.
Urtica urens while Breastfeeding
Stinging nettle (Urtica dioica and Urtica urens) preparations have been used in nursing mothers orally as a postpartum as a "tonic" for treating anemia;[1][2] and is a purported galactogogue;[2][3][4][5][6][7] however, no scientifically valid clinical trials support the safety and efficacy in nursing mothers or infants for any use. Galactogogues should never replace evaluation and counseling on modifiable factors that affect milk production.[8] Although stinging nettle is generally well tolerated in adults, topical use can cause urticaria when applied topically, and application on one mother's nipple resulted in allergic skin rash in her breastfed infant. It is probably best not to apply stinging nettle topically to the breast while breastfeeding. Dietary supplements do not require extensive pre-marketing approval from the U.S. Food and Drug Administration. Manufacturers are responsible to ensure the safety, but do not need to the safety and effectiveness of dietary supplements before they are marketed. Dietary supplements may contain multiple ingredients, and differences are often found between labeled and actual ingredients or their amounts. A manufacturer may contract with an independent organization to verify the quality of a product or its ingredients, but that does certify the safety or effectiveness of a product. Because of the above issues, clinical testing results on one product may not be applicable to other products. More detailed information #about dietary supplements# is available elsewhere on the LactMed Web site.
Thyroid, unspecified while Breastfeeding
CAS Number: 8028-36-2
Thyroid is an animal-derived mixture of levothyroxine (T4) and liothyronine (T3), which are normal components of human milk. Limited data on exogenous replacement doses of levothyroxine during breastfeeding indicate no adverse effects in infants. If thyroid is required by the mother, it is not a reason to discontinue breastfeeding. The American Thyroid Association recommends that subclinical and overt hypothyroidism should be treated with levothyroxine in lactating women seeking to breastfeed.[1] Thyroid dosage requirement may be increased in the postpartum period compared to prepregnancy requirements patients with Hashimoto's thyroiditis.[2]
Arsenic trioxide while Breastfeeding
CAS Number: 1327-53-3
Most sources consider breastfeeding to be contraindicated during maternal antineoplastic drug therapy. It might be possible to breastfeed safely during intermittent therapy with an appropriate period of breastfeeding abstinence; the manufacturer recommends an abstinence period of 1 week after the last dose. Chemotherapy may adversely affect the normal microbiome and chemical makeup of breastmilk.[1] Women who receive chemotherapy during pregnancy are more likely to have difficulty nursing their infant.[2]
Pulsatilla vulgaris while Breastfeeding
Pulsatilla (Anemone pulsatilla and other related species) contains ranunculin, protoanemonin, and anemonin as well as triterpene saponins and flavonoids. The fresh plant is extremely irritating to the skin, gastrointestinal tract and mucous membranes. Allergic reactions have been reported to pulsatilla. Homeopathic preparations of pulsatilla are reportedly used for sore nipples and mastitis,[1] to reduce an overabundant milk supply,[2] or to increase milk supply.[3] Galactogogues should never replace evaluation and counseling on modifiable factors that affect milk production.[4] No scientifically valid clinical trials support either of these uses. Because of a lack of information, other agents may be preferred in nursing mothers. Dietary supplements do not require extensive pre-marketing approval from the U.S. Food and Drug Administration. Manufacturers are responsible to ensure the safety, but do not need to the safety and effectiveness of dietary supplements before they are marketed. Dietary supplements may contain multiple ingredients, and differences are often found between labeled and actual ingredients or their amounts. A manufacturer may contract with an independent organization to verify the quality of a product or its ingredients, but that does certify the safety or effectiveness of a product. Because of the above issues, clinical testing results on one product may not be applicable to other products. More detailed
Ginkgo while Breastfeeding
CAS Number: 90045-36-6
Ginkgo (Ginkgo biloba) leaf contains flavonoids (e.g., quercetin, kaempferol, isorhamnetine) and several terpene trilactones (e.g., ginkgolides, bilobalide) as well as numerous minor components. Standardization is based on ginkgo flavone glycoside and terpenoid content. Raw ginkgo seeds contain potentially toxic cyanogenic glycosides and should not be used; roasted seeds do not carry this risk. Ginkgo has no specific uses during breastfeeding, but is commonly used as an antioxidant, a vasodilator to increase cerebral and peripheral perfusion, and to improve memory. No data exist on the safety and efficacy of ginkgo in nursing mothers or infants. In general, it is well tolerated, but occasionally minor symptoms (e.g., headache, nausea, gastrointestinal complaints, allergic skin rashes) occur in those taking the drug. Ginkgo has caused some cases of bleeding in healthy volunteers caused by its antiplatelet activity. Because there is no published experience with ginkgo during breastfeeding, an alternate therapy may be preferred, especially while nursing a newborn or preterm infant.[1] Dietary supplements do not require extensive pre-marketing approval from the U.S. Food and Drug Administration. Manufacturers are responsible to ensure the safety, but do not need to the safety and effectiveness of dietary supplements before they are marketed. Dietary supplements may contain multiple ingredients, and differences are often found between labeled and actual ingredients or their amounts. A manufacturer may contract with an independent organization to verify the quality of a product or its ingredients, but that does certify the safety or effectiveness of a product. Because of the above issues, clinical testing results on one product may not be applicable to other products. More detailed information #about dietary supplements# is available elsewhere on the LactMed Web site.
Goldenseal while Breastfeeding
CAS Number: 84603-60-1
Goldenseal (Hydrastis canadensis) root contains berberine and other isoquinoline alkaloids. Goldenseal has traditionally been used as an anti-infective both systemically and topically, although high-quality studies of its efficacy and safety are lacking. It has also been used to mask illicit drugs in the urine, although it appears to be ineffective with modern laboratory methods. Goldenseal has been used topically by nursing mothers to treat sore nipples.[1] No data exist on the excretion of any components of goldenseal into breastmilk or on the safety and efficacy of goldenseal in nursing mothers. Berberine can displace bilirubin from serum albumin, causing concern about exposure of newborn infants, because bilirubin can build up in the infant's brain, causing brain damage. However, the extent of berberine's passage from the mother to the infant is unknown. Most sources recommend avoiding exposure of neonates to goldenseal via breastfeeding or otherwise.[2][3][4] Dietary supplements do not require extensive pre-marketing approval from the U.S. Food and Drug Administration. Manufacturers are responsible to ensure the safety, but do not need to the safety and effectiveness of dietary supplements before they are marketed. Dietary supplements may contain multiple ingredients, and differences are often found between labeled and actual ingredients or their amounts. A manufacturer may contract with an independent organization to verify the quality of a product or its ingredients, but that does certify the safety or effectiveness of a product. Because of the above issues, clinical testing results on one product may not be applicable to other products. More detailed information #about dietary supplements# is available elsewhere on the LactMed Web site.
Pollen Weeds Breastfeeding Analsys - 3
Urtica urens and Breastfeeding
Low RiskWarning: Best not to apply Urtica Urens topically to the breast while breastfeeding
Thyroid, unspecified and Breastfeeding
SafeBaptisia tinctoria root and Breastfeeding
UnsafeWild indigo is an herb. The root is used to make medicine.Wild indigo is used for infections such as diphtheria, influenza (flu), swine flu, the common cold and other upper respiratory tract infections, lymph node infections, scarlet fever, malaria, and typhoid. It is also used for sore tonsils (tonsillitis), sore throat, swelling of the mouth and throat, fever, boils, and Crohns disease. Some people apply wild indigo directly to the skin for ulcers, sore and painful nipples, as a douche for vaginal discharge, and for cleaning open and swollen wounds. Wild indigo is UNSAFE when taken by mouth or applied to the skin, long-term or in large doses. Large doses can cause vomiting, diarrhea, other intestinal problems, and spasms.
While breastfeeding wild indigo is likely not safe when taken by mouth or applied to the skin. Avoid use.
Gelsemium sempervirens root and Breastfeeding
UnsafeAll parts of the false jasmine usually contain toxic alkaloids. Eating just one flower has reportedly been lethal to children. The plant can also cause skin allergies in some people and it is possible that the plant toxins can be absorbed through the skin, especially if there are cuts. It�s not recommended to use false jasmine while breastfeeding. It is acceptable in homeopathic preparation.
Pulsatilla vulgaris and Breastfeeding
Low RiskNote: Mostly safe in Homeopathic preparations
Goldenseal and Breastfeeding
UnsafeWhat should I do if already breastfed my kid after using Pollen Weeds?
Due to high dilution of ingredients in homeopathic medicines they do not create much problem for baby. Pollen Weeds is a homeopathic medicine and if your baby does not have any abnormal symptoms then there is nothing to worry about. Be careful with too much usage of ethanol based homeopathic medicines during breastfeeding.
I am nursing mother and my doctor has suggested me to use Pollen Weeds, is it safe?
Homeopathic medicines are usually safe in breastfeeding and if Pollen Weeds has been recommended by doctor then there should be no concern about its usage in breastfeeding.
If I am using Pollen Weeds, will my baby need extra monitoring?
Not exactly.
Who can I talk to if I have questions about usage of Pollen Weeds in breastfeeding?
US
National Womens Health and Breastfeeding Helpline: 800-994-9662 (TDD 888-220-5446) 9 a.m. and 6 p.m. ET, Monday through Friday
UK
National Breastfeeding Helpline: 0300-100-0212 9.30am to 9.30pm, daily
Association of Breastfeeding Mothers: 0300-330-5453
La Leche League: 0345-120-2918
The Breastfeeding Network supporter line in Bengali and Sylheti: 0300-456-2421
National Childbirth Trust (NCT): 0300-330-0700
Australia
National Breastfeeding Helpline: 1800-686-268 24 hours a day, 7 days a week
Canada
Telehealth Ontario for breastfeeding: 1-866-797-0000 24 hours a day, 7 days a week
