Daytime/nitetime Severe Cold And Flu Maximum Strength while Breastfeeding
What is Daytime/nitetime Severe Cold And Flu Maximum Strength used for?
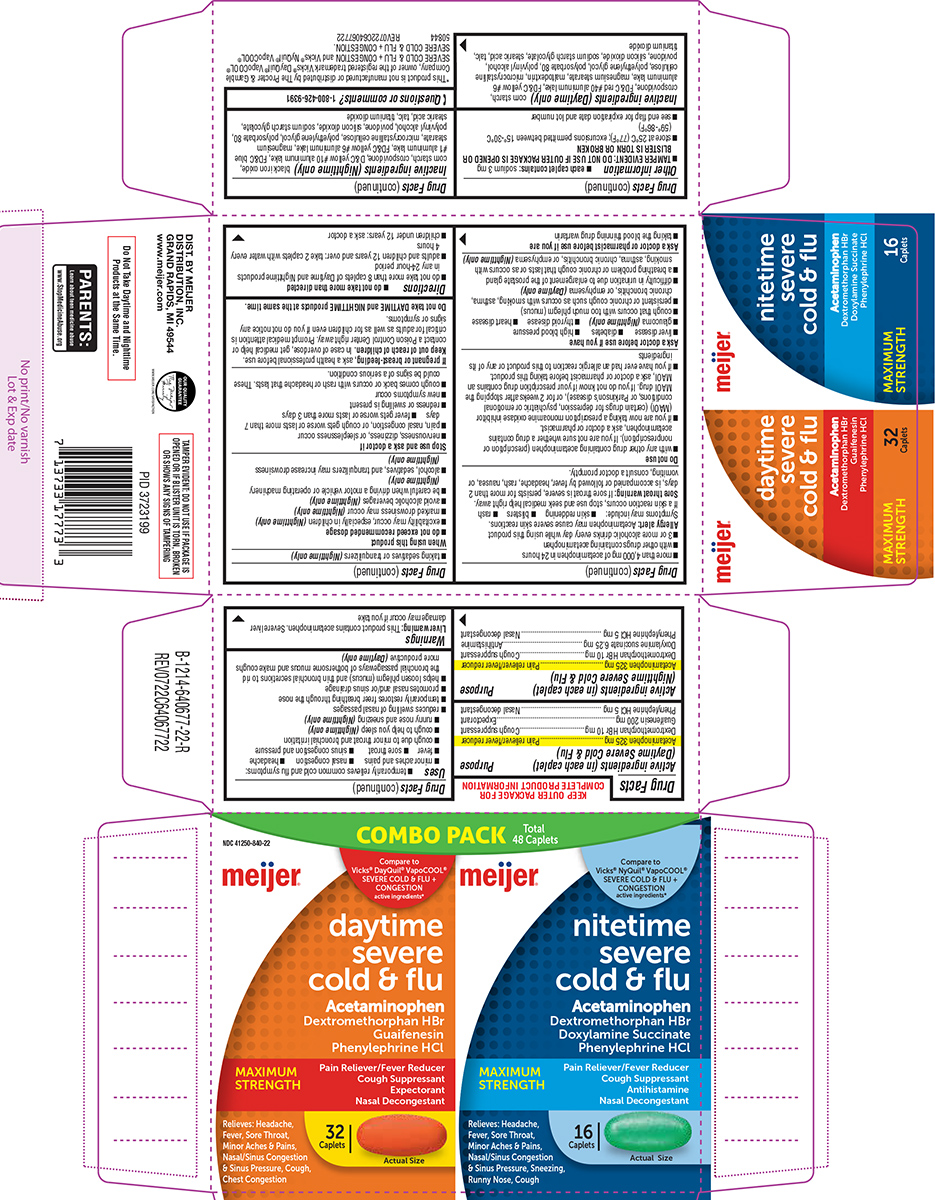
Purpose: Pain reliever/fever reducer Cough suppressant Expectorant Nasal decongestant
Purpose Pain reliever/fever reducer Cough suppressant Antihistamine Nasal decongestant
Is Daytime/nitetime Severe Cold And Flu Maximum Strength safe to use while breastfeeding? Can it interfere with growth and development of my kid?

Daytime/nitetime Severe Cold And Flu Maximum Strength Breastfeeding Analsys
Acetaminophen while Breastfeeding
SafeCAS Number: 103-90-2
Excreted in very low amount into breast milk. Infant intake may be lower than 4% of usual pediatric dose. The American Academy of Pediatrics rates it as compatible with Breastfeeding.
Dextromethorphan hydrobromide while Breastfeeding
SafeCAS Number: 125-71-3
Cough suppressant related with morphine and codeine which is lacking of analgesic or sedative properties. Commonly prescribed by pediatricians. On latest update relevant data on breastfeeding was not found. Because reported low toxicity and mild side effect it is considered to be safe while breastfeeding. Frequently associated to caffeine and other products that are usually compatible with breastfeeding. Avoid use of multiple drug and alcohol containing medication.
Guaifenesin while Breastfeeding
Low RiskCAS Number: 93-14-1
It is used as an expectorant, alone or in association with other products. Proofs on its effectiveness are sparse. In some instances, preparations of Guaifenesin may contain alcohol as excipient with a concentration as high as 5%. At latest update, relevant published data on excretion into breast milk were not found. Until more information on this medication is available, other option known to be safer would be recommended, mostly in the post-natal period or in cases of prematurity. If used while breastfeeding, a moderate use with the lowest dose as possible and avoiding those preparations with alcoholic excipient, should be preferred. Because effectiveness is poor and likelihood of side effects does exist, especially in multi-association, the US Agency for Drug Administration (FDA) is currently doing efforts for discontinuation of this and others at-the-counter products, that are formulated for cough relief (Guaifenesin, Dextromethorphan, Phenylephrine, Pseudoephedrine, Brompheniramine, etc.)
Phenylephrine hydrochloride while Breastfeeding
Low RiskCAS Number: 59-42-7
Used on topical decongestant solutions for nose drops at low concentration. 10% midriatic eye drops are available. Because low concentration is used on nose and ophtalmic drops a significant excretion into breast milk is unlikely. Low oral biodisponibility minimizes any risk of harmful effect in the infant. Authorized for nasal or ophtalmic use on children aged younger than 1 year. Although on latest update relevant data on breastfeeding was not found it is considered to be safe when minimal dose is used. Avoid excessive or long term use. A related drug Pseudoephedrine can inhibit milk production. It would be advisable to press on the lachrimal sac to minimize absorption.
Acetaminophen while Breastfeeding
SafeCAS Number: 103-90-2
Excreted in very low amount into breast milk. Infant intake may be lower than 4% of usual pediatric dose. The American Academy of Pediatrics rates it as compatible with Breastfeeding.
Dextromethorphan hydrobromide while Breastfeeding
SafeCAS Number: 125-71-3
Cough suppressant related with morphine and codeine which is lacking of analgesic or sedative properties. Commonly prescribed by pediatricians. On latest update relevant data on breastfeeding was not found. Because reported low toxicity and mild side effect it is considered to be safe while breastfeeding. Frequently associated to caffeine and other products that are usually compatible with breastfeeding. Avoid use of multiple drug and alcohol containing medication.
Doxylamine succinate while Breastfeeding
UnsafeCAS Number: 469-21-6
It is a first generation antihistamine drug which is related to ethanolamine, with sedative and anti-muscarinic effects. It has been used as hypnotic and for vomiting relief. At latest update, relevant published data on excretion into breast milk were not found. Pharmacokinetic characteristics would favour that it may be excreted into breast milk in significant amount. On a telephone survey, 10% of infants whose mothers were on several types of antihistamine medication have suffered of colicky pain and irritability that disappeared without treatment. For both treatment of mothers and infants would be safer the use of tested antihistamine medication without sedative effect, especially in prematures and infants younger than 1 month of age. Whenever used while breastfeeding, the use of the lower effective dose and for the shortest time as possible is recommended. Follow-up for somnolence and feeding troubles should be warranted. Bed-sharing is not recommended when the mother is on this medication.
Phenylephrine hydrochloride while Breastfeeding
Low RiskCAS Number: 59-42-7
Used on topical decongestant solutions for nose drops at low concentration. 10% midriatic eye drops are available. Because low concentration is used on nose and ophtalmic drops a significant excretion into breast milk is unlikely. Low oral biodisponibility minimizes any risk of harmful effect in the infant. Authorized for nasal or ophtalmic use on children aged younger than 1 year. Although on latest update relevant data on breastfeeding was not found it is considered to be safe when minimal dose is used. Avoid excessive or long term use. A related drug Pseudoephedrine can inhibit milk production. It would be advisable to press on the lachrimal sac to minimize absorption.
Daytime/nitetime Severe Cold And Flu Maximum Strength Breastfeeding Analsys - 2
Acetaminophen while Breastfeeding
CAS Number: 103-90-2
Acetaminophen is a good choice for analgesia, and fever reduction in nursing mothers. Amounts in milk are much less than doses usually given to infants. Adverse effects in breastfed infants appear to be rare.
Dextromethorphan hydrobromide while Breastfeeding
CAS Number: 125-71-3
Neither the excretion of dextromethorphan in milk nor its effect on breastfed infants have been studied. It is unlikely that with usual maternal doses amounts in breastmilk would harm the nursing infant, especially in infants over 2 months of age. It is best to avoid the use of products with a high alcohol content while nursing.
Guaifenesin while Breastfeeding
CAS Number: 93-14-1
Neither the excretion of guaifenesin in milk nor its effect on breastfed infants have been studied. It is unlikely that with usual maternal doses amounts in breastmilk would harm the nursing infant, especially in infants over 2 months of age. It is best to avoid the use of products with a high alcohol content while nursing.
Phenylephrine hydrochloride while Breastfeeding
CAS Number: 59-42-7
The oral bioavailability of phenylephrine is only about 40%,[1] so the drug is unlikely to reach the infant in large amounts. However, intravenous or oral administration of phenylephrine might decrease milk production. Because no information is available on the use of oral phenylephrine during breastfeeding, an alternate drug may be preferred, especially while nursing a newborn or preterm infant.Phenylephrine nasal spray or ophthalmic drops are less likely to decrease lactation. To substantially diminish the effect of the drug after using eye drops, place pressure over the tear duct by the corner of the eye for 1 minute or more, then remove the excess solution with an absorbent tissue.
Acetaminophen while Breastfeeding
CAS Number: 103-90-2
Acetaminophen is a good choice for analgesia, and fever reduction in nursing mothers. Amounts in milk are much less than doses usually given to infants. Adverse effects in breastfed infants appear to be rare.
Dextromethorphan hydrobromide while Breastfeeding
CAS Number: 125-71-3
Neither the excretion of dextromethorphan in milk nor its effect on breastfed infants have been studied. It is unlikely that with usual maternal doses amounts in breastmilk would harm the nursing infant, especially in infants over 2 months of age. It is best to avoid the use of products with a high alcohol content while nursing.
Doxylamine succinate while Breastfeeding
CAS Number: 469-21-6
Small occasional doses of doxylamine would not be expected to cause any adverse effects in breastfed infants. Larger doses or more prolonged use may cause drowsiness and other effects in the infant or decrease the milk supply, particularly in combination with a sympathomimetic such as pseudoephedrine or before lactation is well established.
Phenylephrine hydrochloride while Breastfeeding
CAS Number: 59-42-7
The oral bioavailability of phenylephrine is only about 40%,[1] so the drug is unlikely to reach the infant in large amounts. However, intravenous or oral administration of phenylephrine might decrease milk production. Because no information is available on the use of oral phenylephrine during breastfeeding, an alternate drug may be preferred, especially while nursing a newborn or preterm infant.Phenylephrine nasal spray or ophthalmic drops are less likely to decrease lactation. To substantially diminish the effect of the drug after using eye drops, place pressure over the tear duct by the corner of the eye for 1 minute or more, then remove the excess solution with an absorbent tissue.
What should I do if already breastfed my kid after using Daytime/nitetime Severe Cold And Flu Maximum Strength?
We have already established that Daytime/nitetime Severe Cold And Flu Maximum Strength is unsafe in breastfeeding and breastfeeding while using Daytime/nitetime Severe Cold And Flu Maximum Strength is not a good idea however if have already used
My health care provider has asked me to use Daytime/nitetime Severe Cold And Flu Maximum Strength, what to do?
If your doctor knows that you are breastfeeding mother and still prescribes Daytime/nitetime Severe Cold And Flu Maximum Strength then there must be good reason for that as Daytime/nitetime Severe Cold And Flu Maximum Strength is considered unsafe, It usually happens when doctor finds that overall advantage of taking
If I am using Daytime/nitetime Severe Cold And Flu Maximum Strength, will my baby need extra monitoring?
Yes, Extra monitoring is required if mother is using Daytime/nitetime Severe Cold And Flu Maximum Strength and breastfeeding as it is considered unsafe for baby.
Who can I talk to if I have questions about usage of Daytime/nitetime Severe Cold And Flu Maximum Strength in breastfeeding?
US
National Womens Health and Breastfeeding Helpline: 800-994-9662 (TDD 888-220-5446) 9 a.m. and 6 p.m. ET, Monday through Friday
UK
National Breastfeeding Helpline: 0300-100-0212 9.30am to 9.30pm, daily
Association of Breastfeeding Mothers: 0300-330-5453
La Leche League: 0345-120-2918
The Breastfeeding Network supporter line in Bengali and Sylheti: 0300-456-2421
National Childbirth Trust (NCT): 0300-330-0700
Australia
National Breastfeeding Helpline: 1800-686-268 24 hours a day, 7 days a week
Canada
Telehealth Ontario for breastfeeding: 1-866-797-0000 24 hours a day, 7 days a week
