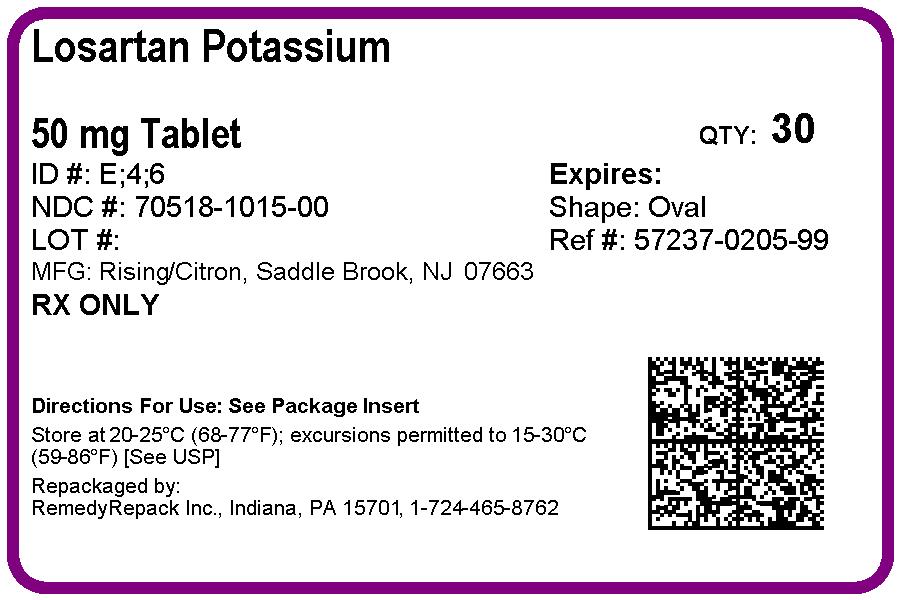
Modern medicine has evolved so much so that sooner or later every breastfeeding mother needs to take it in one form or other. Medication that is present in mothers blood will transfer into her breast milk to some extent. Most drugs do so at low levels and pose no real risk to infants but then there are some exceptions. In This post will discuss whether Remedyrepack Inc. Losartan Potassium Tablet, Film Coated 50 Mg is safe in breast-feeding or not.
What is Remedyrepack Inc. Losartan Potassium Tablet, Film Coated 50 Mg used for?
Losartan potassium tablets are an angiotensin II receptor blocker (ARB) indicated for: Treatment of hypertension, to lower blood pressure in adults and children greater than 6 years old. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. ( 1.1) Reduction of the risk of stroke in patients with hypertension and left ventricular hypertrophy. There is evidence that this benefit does not apply to Black patients. ( 1.2) Treatment of diabetic nephropathy with an elevated serum creatinine and proteinuria in patients with type 2 diabetes and a history of hypertension. ( 1.3) 1.1 Hypertension Losartan potassium tablets are indicated for the treatment of hypertension in adults and pediatric patients 6 years of age and older, to lower blood pressure. Lowering blood pressure lowers the risk of fatal and nonfatal cardiovascular (CV) events, primarily strokes and myocardial infarction. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including losartan. Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than 1 drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program’s Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC). Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly. Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal. Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in Black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy. Losartan potassium tablets may be administered with other antihypertensive agents. 1.2 Hypertensive Patients with Left Ventricular Hypertrophy Losartan potassium tablets are indicated to reduce the risk of stroke in patients with hypertension and left ventricular hypertrophy, but there is evidence that this benefit does not apply to Black patients [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)]. 1.3 Nephropathy in Type 2 Diabetic Patients Losartan potassium tablets are indicated for the treatment of diabetic nephropathy with an elevated serum creatinine and proteinuria (urinary albumin to creatinine ratio ≥300 mg/g) in patients with type 2 diabetes and a history of hypertension. In this population, losartan potassium tablets reduces the rate of progression of nephropathy as measured by the occurrence of doubling of serum creatinine or end stage renal disease (need for dialysis or renal transplantation) [see Clinical Studies (14.3)].
Can I continue breastfeeding if I am using Remedyrepack Inc. Losartan Potassium Tablet, Film Coated 50 Mg? How long does it stays in breast milk?
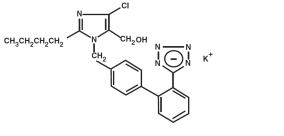
Losartan potassium is the one and only active ingredient present in Remedyrepack Inc. Losartan Potassium Tablet, Film Coated 50 Mg. Losartan potassium in itself is a low risk drug for lactation so it is easy to understand that Remedyrepack Inc. Losartan Potassium Tablet, Film Coated 50 Mg also comes in category of Low Risk item while breastfeeding. Below is the summary of Losartan potassium in breastfeeding.
Statement of Manufacturer/Labeler about breastfeeding usage
8.3 Nursing Mothers It is not known whether losartan is excreted in human milk, but significant levels of losartan and its active metabolite were shown to be present in rat milk. Because of the potential for adverse effects on the nursing infant, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Remedyrepack Inc. Losartan Potassium Tablet, Film Coated 50 Mg Breastfeeding Analsys
Low RiskCAS Number: 124750-99-8
At latest update, relevant published data on excretion into breast milk were not found. A high protein-binding capacity makes excretion into breast milk unlikely. In addition, a low oral bioavailability makes difficult the absorption towards the infant's plasma from ingested milk, except in prematures or newborns who may show an increased absorption. Case report of kidney function impairment of a baby whose mother had taken Telmisartan in pregnancy. Until more data on this medication is available, safer alternative drugs are preferred, especially in premature babies or during the neonatal period. Should an ARA-II medication (Sartan type) be necessary, the associated risk may be decreased by choosing the one with a favorable pharmacokinetics (shorter half-life elimination time and lower bioavailability) like Eprosartan and Losartan
Remedyrepack Inc. Losartan Potassium Tablet, Film Coated 50 Mg Breastfeeding Analsys - 2
CAS Number: 114798-26-4
Because no information is available on the use of losartan during breastfeeding, an alternate drug may be preferred, especially while nursing a newborn or preterm infant.
I am nursing mother and I have already used Remedyrepack Inc. Losartan Potassium Tablet, Film Coated 50 Mg, what should I do?
During whole lactation period you shall first discuss with your doctor and then together you shall decide whether you shall take that drug or not however if you have already taken Remedyrepack Inc. Losartan Potassium Tablet, Film Coated 50 Mg then you shall inform your doctor, But you should not be worried too much as Remedyrepack Inc. Losartan Potassium Tablet, Film Coated 50 Mg comes in category of low risk drug.
My doctor has prescribed me Remedyrepack Inc. Losartan Potassium Tablet, Film Coated 50 Mg, what should I do?
Though Remedyrepack Inc. Losartan Potassium Tablet, Film Coated 50 Mg dose not comes in category of safe drugs rather it comes in category of low risk but if your doctor is aware that you are breastfeeding your baby and has still recommended it then its advantages must be outweighing the risks.
If I am using Remedyrepack Inc. Losartan Potassium Tablet, Film Coated 50 Mg, will my baby need extra monitoring?
Not much
Who can I talk to if I have questions about usage of Remedyrepack Inc. Losartan Potassium Tablet, Film Coated 50 Mg in breastfeeding?
US
National Womens Health and Breastfeeding Helpline: 800-994-9662 (TDD 888-220-5446) 9 a.m. and 6 p.m. ET, Monday through Friday
UK
National Breastfeeding Helpline: 0300-100-0212 9.30am to 9.30pm, daily
Association of Breastfeeding Mothers: 0300-330-5453
La Leche League: 0345-120-2918
The Breastfeeding Network supporter line in Bengali and Sylheti: 0300-456-2421
National Childbirth Trust (NCT): 0300-330-0700
Australia
National Breastfeeding Helpline: 1800-686-268 24 hours a day, 7 days a week
Canada
Telehealth Ontario for breastfeeding: 1-866-797-0000 24 hours a day, 7 days a week