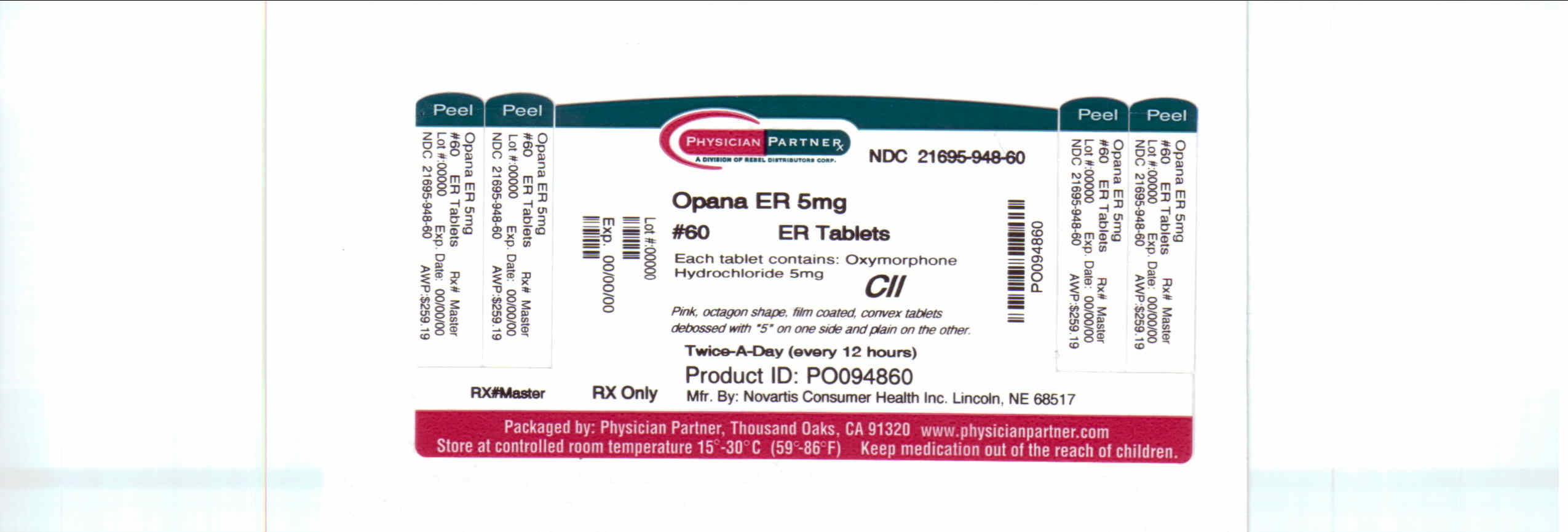
Opana Er | Oxymorphone Hydrochloride Tablet, Film Coated, Extended Release while Breastfeeding
What is Opana Er | Oxymorphone Hydrochloride Tablet, Film Coated, Extended Release ?
Is using Opana Er | Oxymorphone Hydrochloride Tablet, Film Coated, Extended Release safe or dangerous while breastfeeding?

Nursing Mothers It is not known whether oxymorphone is excreted in human milk. Because many drugs, including some opioids, are excreted in human milk, caution should be exercised when OPANA ER is administered to a nursing woman. Ordinarily, nursing should not be undertaken while a patient is receiving oxymorphone because of the possibility of sedation and/or respiratory depression in the infant.
Opana Er | Oxymorphone Hydrochloride Tablet, Film Coated, Extended Release Breastfeeding Analsys
Oxymorphone hydrochloride while Breastfeeding
CAS Number: 76-41-5
No data are available on the use of oxymorphone during breastfeeding. Maternal use of oral narcotics during breastfeeding can cause infant drowsiness, central nervous system depression and even death. Newborn infants seem to be particularly sensitive to the effects of even small dosages of narcotic analgesics. Once the mother's milk comes in, it is best to provide pain control with a nonnarcotic analgesic and limit maternal intake of oral oxymorphone to a few days at low dosages, with close infant monitoring. If the baby shows signs of increased sleepiness (more than usual), difficulty breastfeeding, breathing difficulties, or limpness, a physician should be contacted immediately. Other agents are preferred over oxymorphone during breastfeeding.
Opana Er | Oxymorphone Hydrochloride Tablet, Film Coated, Extended Release Breastfeeding Analsys - 2
Oxymorphone hydrochloride and Breastfeeding
Dangerous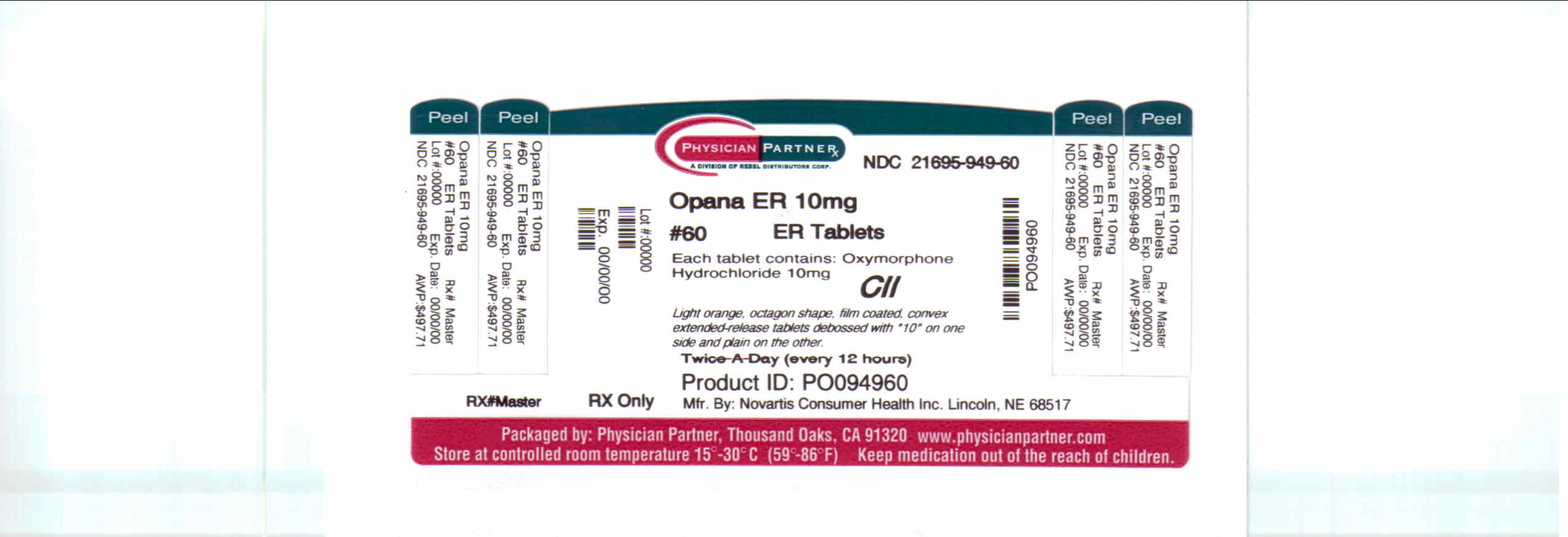
What should I do if already breastfed my kid after using Opana Er | Oxymorphone Hydrochloride Tablet, Film Coated, Extended Release?
You should immediately inform your health care provider about Opana Er | Oxymorphone Hydrochloride Tablet, Film Coated, Extended Release usage and your breastfeeding interval after usage of
My doctor has prescribed me Opana Er | Oxymorphone Hydrochloride Tablet, Film Coated, Extended Release, what should I do?
Please double check with your doctor if he is aware of your breastfeeding stratus, Ask your doctor if there is any safe alternative of Opana Er | Oxymorphone Hydrochloride Tablet, Film Coated, Extended Release. Check with your doctor if you shall temporally stop breastfeeding. You may go for second opinion as well. Still after all of this if your doctor still recommends Opana Er | Oxymorphone Hydrochloride Tablet, Film Coated, Extended Release then go for it as they have access on more detailed medical and scientific information and they understand your individual medical situation much better.
If I am using Opana Er | Oxymorphone Hydrochloride Tablet, Film Coated, Extended Release, will my baby need extra monitoring?
Extreme level of monitoring required as Opana Er | Oxymorphone Hydrochloride Tablet, Film Coated, Extended Release could be dangerous for kid.
Who can I talk to if I have questions about usage of Opana Er | Oxymorphone Hydrochloride Tablet, Film Coated, Extended Release in breastfeeding?
US
National Womens Health and Breastfeeding Helpline: 800-994-9662 (TDD 888-220-5446) 9 a.m. and 6 p.m. ET, Monday through Friday
UK
National Breastfeeding Helpline: 0300-100-0212 9.30am to 9.30pm, daily
Association of Breastfeeding Mothers: 0300-330-5453
La Leche League: 0345-120-2918
The Breastfeeding Network supporter line in Bengali and Sylheti: 0300-456-2421
National Childbirth Trust (NCT): 0300-330-0700
Australia
National Breastfeeding Helpline: 1800-686-268 24 hours a day, 7 days a week
Canada
Telehealth Ontario for breastfeeding: 1-866-797-0000 24 hours a day, 7 days a week
