Most health expert recommend six month of exclusive breastfeeding but statics suggest that numbers are not good, almost 95% mothers start breastfeeding but this number drops to 40% in first three month and further it drops to 15% till fifth month. Sometime its due to need of medication usage. Because of these statics its important to provide good information on safety of drugs in breastfeeding so that it can be improved when possible. In this FAQ sheet we will discuss about exposure to Agenerase | Amprenavir Solution while breastfeeding. We will also discuss about common side effects and warnings associated with Agenerase | Amprenavir Solution.
What is Agenerase | Amprenavir Solution used for?
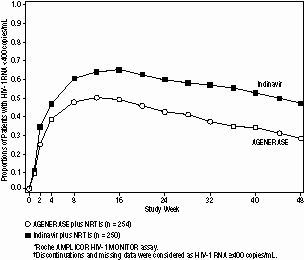
AGENERASE ( amprenavir ) is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection. The following points should be considered when initiating therapy with AGENERASE: In a study of NRTI-experienced, protease inhibitor-naive patients, AGENERASE was found to be significantly less effective than indinavir (see Description of Clinical Studies). Mild to moderate gastrointestinal adverse events led to discontinuation of AGENERASE primarily during the first 12 weeks of therapy (see ADVERSE REACTIONS). There are no data on response to therapy with AGENERASE in protease inhibitor-experienced patients. AGENERASE Oral Solution should be used only when AGENERASE Capsules or other protease inhibitor formulations are not therapeutic options. Description of Clinical Studies Therapy-Naive Adults: PROAB3001, a randomized, double-blind, placebo-controlled, multicenter study, compared treatment with AGENERASE Capsules (1,200 mg twice daily) plus lamivudine (150 mg twice daily) plus zidovudine (300 mg twice daily) versus lamivudine (150 mg twice daily) plus zidovudine (300 mg twice daily) in 232 patients. Through 24 weeks of therapy, 53% of patients assigned to AGENERASE/zidovudine/lamivudine achieved HIV-1 RNA <400 copies/mL. Through Week 48, the antiviral response was 41%. Through 24 weeks of therapy, 11% of patients assigned to zidovudine/lamivudine achieved HIV-1 RNA <400 copies/mL. Antiviral response beyond Week 24 is not interpretable because the majority of patients discontinued or changed their antiretroviral therapy. NRTI-Experienced Adults: PROAB3006, a randomized, open-label multicenter study, compared treatment with AGENERASE Capsules (1,200 mg twice daily) plus NRTIs versus indinavir (800 mg every 8 hours) plus NRTIs in 504 NRTI-experienced, protease inhibitor-naive patients, median age 37 years (range 20 to 71 years), 72% Caucasian, 80% male, with a median CD4 cell count of 404 cells/mm3 (range 9 to 1,706 cells/mm3) and a median plasma HIV-1 RNA level of 3.93 log10 copies/mL (range 2.60 to 7.01 log10 copies/mL) at baseline. Through 48 weeks of therapy, the median CD4 cell count increase from baseline in the amprenavir group was significantly lower than in the indinavir group, 97 cells/mm3 versus 144 cells/mm3, respectively. There was also a significant difference in the proportions of patients with plasma HIV-1 RNA levels <400 copies/mL through 48 weeks (see Figure 1 and Table 5). Figure 1. Virologic Response Through Week 48, PROAB3006 *,† HIV-1 RNA status and reasons for discontinuation of randomized treatment at 48 weeks are summarized (Table 5). Table 5. Outcomes of Randomized Treatment Through Week 48 (PROAB3006) Outcome AGENERASE (n = 254) Indinavir (n = 250) HIV-1 RNA <400 copies/mL* 30% 49% HIV-1 RNA ≥400 copies/mL†,‡ 38% 26% Discontinued due to adverse events*,‡ 16% 12% Discontinued due to other reasons‡,§ 16% 13% * Corresponds to rates at Week 48 in Figure 1. †Virological failures at or before Week 48. ‡Considered to be treatment failure in the analysis. §Includes discontinuations due to consent withdrawn, loss to follow-up, protocol violations, non-compliance, pregnancy, never treated, and other reasons. Figure 1. Virologic Response Through Week 48, PROAB3006
Agenerase | Amprenavir Solution while breastfeeding safe or not? Can there be any side effects for infant while using it during breastfeeding?
As Agenerase | Amprenavir Solution is made of only Amprenavir, and Amprenavir is unsafe to use in breastfeeding we can safely reach on conclusion that Agenerase | Amprenavir Solution is also unsafe to use while breastfeeding. Below is detailed analysis of Amprenavir and Agenerase | Amprenavir Solution during location. We recommend you to go through provided detailed analysis as below take decision accordingly. We also recommend you talk to your health care provider before making final decision.
Statement of Manufacturer/Labeler about breastfeeding usage
Nursing Mothers The Centers for Disease Control and Prevention recommend that HIV-infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV. Although it is not known if amprenavir is excreted in human milk, amprenavir is secreted into the milk of lactating rats. Because of both the potential for HIV transmission and the potential for serious adverse reactions in nursing infants, mothers should be instructed not to breastfeed if they are receiving AGENERASE.
Agenerase | Amprenavir Solution Breastfeeding Analsys
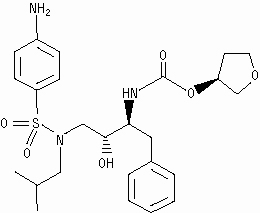
UnsafeCAS Number: 161814-49-9
Anti-HIV drug. No experience in young infants. Withdrawn from market. Mothers should be aware of HIV transmission through breast milk has been reported.
What should I do if already breastfed my kid after using Agenerase | Amprenavir Solution?
We have already established that Agenerase | Amprenavir Solution is unsafe in breastfeeding and breastfeeding while using Agenerase | Amprenavir Solution is not a good idea however if have already used and then breastfed the baby then you shall monitor the behavior and health of baby closely and inform your doctor as soon as possible. In case of emergency please call 911.
I am nursing mother and my doctor has suggested me to use Agenerase | Amprenavir Solution, is it safe?
If your doctor knows that you are breastfeeding mother and still prescribes Agenerase | Amprenavir Solution then there must be good reason for that as Agenerase | Amprenavir Solution is considered unsafe, It usually happens when doctor finds that overall advantage of taking outweighs the overall risk.
If I am using Agenerase | Amprenavir Solution, will my baby need extra monitoring?
Yes, Extra monitoring is required if mother is using Agenerase | Amprenavir Solution and breastfeeding as it is considered unsafe for baby.
Who can I talk to if I have questions about usage of Agenerase | Amprenavir Solution in breastfeeding?
US
National Womens Health and Breastfeeding Helpline: 800-994-9662 (TDD 888-220-5446) 9 a.m. and 6 p.m. ET, Monday through Friday
UK
National Breastfeeding Helpline: 0300-100-0212 9.30am to 9.30pm, daily
Association of Breastfeeding Mothers: 0300-330-5453
La Leche League: 0345-120-2918
The Breastfeeding Network supporter line in Bengali and Sylheti: 0300-456-2421
National Childbirth Trust (NCT): 0300-330-0700
Australia
National Breastfeeding Helpline: 1800-686-268 24 hours a day, 7 days a week
Canada
Telehealth Ontario for breastfeeding: 1-866-797-0000 24 hours a day, 7 days a week